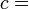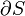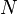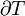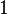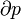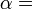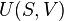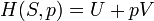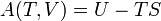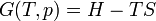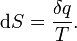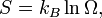Introduction to entropy
Did you know...
This Wikipedia selection is available offline from SOS Children for distribution in the developing world. SOS Children works in 45 African countries; can you help a child in Africa?
| Thermodynamics | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 The classical Carnot heat engine
|
|||||||||||||||||||||
|
Branches
|
|||||||||||||||||||||
|
Systems
State:
Equation of state Ideal gas · Real gas Phase of matter · Equilibrium Control volume · Instruments Processes: Isobaric · Isochoric · Isothermal Adiabatic · Isentropic · Isenthalpic Quasistatic · Polytropic Free expansion Reversibility · Irreversibility Endoreversibility Cycles: Heat engines · Heat pumps Thermal efficiency |
|||||||||||||||||||||
|
System properties
Property diagrams
Intensive and extensive properties Functions of state: Temperature / Entropy (intro.) † Pressure / Volume † Chemical potential / Particle no. † († Conjugate variables) Vapor quality Reduced properties Process functions: Work · Heat |
|||||||||||||||||||||
|
Material properties
|
|||||||||||||||||||||
|
Equations
Carnot's theorem · Clausius theorem · Fundamental relation · Ideal gas law · Maxwell relations · Onsager reciprocal relations · Bridgman's thermodynamic equations
Table of thermodynamic equations |
|||||||||||||||||||||
|
Potentials
|
|||||||||||||||||||||
|
History and culture
Philosophy:
Entropy and time · Entropy and life Brownian ratchet Maxwell's demon Heat death paradox Loschmidt's paradox Synergetics History: General · Heat · Entropy · Gas laws Perpetual motion Theories: Caloric theory · Vis viva Theory of heat Mechanical equivalent of heat Motive power Publications: " An Experimental Enquiry Concerning ... Heat" " On the Equilibrium of Heterogeneous Substances" "Reflections on the Motive Power of Fire" Timelines of: Thermodynamics · Heat engines Art: Maxwell's thermodynamic surface Education: Entropy as energy dispersal |
|||||||||||||||||||||
Entropy is a measure of how evenly energy is distributed in a system. In a physical system entropy provides a measure of the amount of energy that cannot be used to do work.
When heat flows from a hot region to a cold region entropy increases, as heat is distributed throughout the system. The concept of entropy is central to the second law of thermodynamics. The second law determines which physical processes can occur. For example, it predicts that heat flows from high temperature to low temperature in spontaneous processes. The second law of thermodynamics can be stated as saying that the entropy of an isolated system always increases, and processes which increase entropy can occur spontaneously. Since entropy increases as uniformity increases, the second law says qualitatively that uniformity increases.
The term entropy was coined in 1865 by the German physicist Rudolf Clausius, from the Greek words en-, "in", and trope "a turning", in analogy with energy.
Explanation
The concept of thermodynamic entropy arises from the second law of thermodynamics. It uses entropy to quantify the capacity of a system for change, namely that heat flows from a region of higher temperature to one with lower temperature, and to determine whether a thermodynamic process may occur.
Entropy is defined by two descriptions, first as macroscopic relationship between heat flow into a system and the system's change in temperature, and second, on a microscopic level, as the natural logarithm of the number of microstates of a system.
Following the formalism of Clausius, the first definition can be mathematically stated as:
Where dS is the change in entropy and δq is the heat added to the system reversibly. If the temperature is allowed to vary the equation must be integrated over the temperature path. This definition of entropy does not allow the determination of an absolute value, only of differences.
The second definition of entropy comes from statistical mechanics. The entropy of a particular macrostate is defined to be Boltzmann constant times the natural logarithm of the number of microstates corresponding to that macrostate, or mathematically
Where S is the entropy, k is the Boltzmann constant, and omega is the number of microstates.
The macrostate of a system is what we know about the system, for example the temperature, pressure, and volume of a gas in a box. For each set of values of temperature, pressure, and volume there are many arrangements of molecules which result in those values. The number of arrangements of molecules which could result in the same values for temperature, pressure and volume is the number of microstates.
The concept of energy is related to the first law of thermodynamics, which deals with the conservation of energy and under which the loss in heat will result in a decrease in the internal energy of the thermodynamic system. Thermodynamic entropy provides a comparative measure of the amount of this decrease in internal energy of the system and the corresponding increase in internal energy of the surroundings at a given temperature. A simple and more concrete visualization of the second law is that energy of all types changes from being localized to becoming dispersed or spread out, if it is not hindered from doing so. Entropy change is the quantitative measure of that kind of a spontaneous process: how much energy has flowed or how widely it has become spread out at a specific temperature.
Entropy has been developed to describe any of several phenomena, depending on the field and the context in which it is being used. Information entropy takes the mathematical concepts of statistical thermodynamics into areas of probability theory unconnected with heat and energy.
Example of increasing entropy
Ice melting provides an example in which entropy increases in a small system, a thermodynamic system consisting of the surroundings (the warm room) and the entity of glass container, ice, water which has been allowed to reach thermodynamic equilibrium at the melting temperature of ice. In this system, some heat (δQ) from the warmer surroundings at 298 K (77°F, 25°C) transfers to the cooler system of ice and water at its constant temperature (T) of 273 K (32°F, 0°C), the melting temperature of ice. The entropy of the system, which is δQ/T, increases by δQ/273K. The heat δQ for this process is the energy required to change water from the solid state to the liquid state, and is called the enthalpy of fusion, i.e. ΔH for ice fusion.
It is important to realize that the entropy of the surrounding room decreases less than the entropy of the ice and water increases: the room temperature of 298 K is larger than 273 K and therefore the ratio, (entropy change), of δQ/298K for the surroundings is smaller than the ratio (entropy change), of δQ/273K for the ice and water system. This is always true in spontaneous events in a thermodynamic system and it shows the predictive importance of entropy: the final net entropy after such an event is always greater than was the initial entropy.
As the temperature of the cool water rises to that of the room and the room further cools imperceptibly, the sum of the δQ/T over the continuous range, “at many increments”, in the initially cool to finally warm water can be found by calculus. The entire miniature ‘universe’, i.e. this thermodynamic system, has increased in entropy. Energy has spontaneously become more dispersed and spread out in that ‘universe’ than when the glass of ice + water was introduced and became a 'system' within it.
Origins and uses
Originally, entropy was named to describe the waste heat, or more accurately energy losses, from heat engines and other mechanical devices which could never run with 100% efficiency in converting energy into work. Later, the term came to acquire several additional descriptions as more came to be understood about the behaviour of molecules on the microscopic level. In the late 19th century the word "disorder" was used by Ludwig Boltzmann in developing statistical views of entropy using probability theory to describe the increased molecular movement on the microscopic level. That was before quantum behaviour came to be better understood by Werner Heisenberg and those who followed. Descriptions of thermodynamic (heat) entropy on the microscopic level are found in statistical thermodynamics and statistical mechanics.
For most of the 20th century textbooks tended to describe entropy as "disorder", following Boltzmann's early conceptualisation of the motional energy of molecules. More recently there has been a trend in chemistry and physics textbooks to describe entropy in terms of "dispersal of energy". Entropy can also involve the dispersal of particles, which are themselves energetic. Thus there are instances where both particles and energy disperse at different rates when substances are mixed together.
The mathematics developed in statistical thermodynamics were found to be applicable in other disciplines. In particular, information sciences developed the concept of information entropy where a constant replaces the temperature which is inherent in thermodynamic entropy.
Heat and entropy
At a microscopic level, kinetic energy of molecules is responsible for the temperature of a substance or a system. “Heat” is the kinetic energy of molecules being transferred: when motional energy is transferred from hotter surroundings to a cooler system, faster moving molecules in the surroundings collide with the walls of the system which transfers some of their energy to the molecules of the system and makes them move faster.
- Molecules in a gas like nitrogen at room temperature at any instant are moving at an average speed of nearly 500 miles per hour (210 m/s), repeatedly colliding and therefore exchanging energy so that their individual speeds are always changing, even being motionless for an instant if two molecules with exactly the same speed collide head-on, before another molecule hits them and they race off, as fast as 2500 miles an hour. (At higher temperatures average speeds increase and motional energy becomes proportionately greater.)
- Thus motional molecular energy (‘heat energy’) from hotter surroundings, like faster moving molecules in a flame or violently vibrating iron atoms in a hot plate, will melt or boil a substance (the system) at the temperature of its melting or boiling point. That amount of motional energy from the surroundings that is required for melting or boiling is called the phase change energy, specifically the enthalpy of fusion or of vaporization, respectively. This phase change energy breaks bonds between the molecules in the system (not chemical bonds inside the molecules that hold the atoms together) rather than contributing to the motional energy and making the molecules move any faster – so it doesn’t raise the temperature, but instead enables the molecules to break free to move as a liquid or as a vapor.
- In terms of energy, when a solid becomes a liquid or a liquid a vapor, motional energy coming from the surroundings is changed to ‘ potential energy ‘ in the substance (phase change energy, which is released back to the surroundings when the surroundings become cooler than the substance's boiling or melting temperature, respectively). Phase change energy increases the entropy of a substance or system because it is energy that must be spread out in the system from the surroundings so that the substance can exist as a liquid or vapor at a temperature above its melting or boiling point. When this process occurs in a ‘universe’ that consists of the surroundings plus the system, the total energy of the universe becomes more dispersed or spread out as part of the greater energy that was only in the hotter surroundings transfers so that some is in the cooler system. This energy dispersal increases the entropy of the 'universe'.
The important overall principle is that ”Energy of all types changes from being localized to becoming dispersed or spread out, if it is not hindered from doing so. Entropy (or better, entropy change) is the quantitative measure of that kind of a spontaneous process: how much energy has been transferred/T or how widely it has become spread out at a specific temperature.
Classical calculation of entropy
When entropy was first defined and used in 1865 the very existence of atoms was still controversial and there was no concept that temperature was due to the motional energy of molecules or that “heat” was actually the transferring of that motional molecular energy from one place to another. Entropy change, 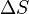 , was described in macroscopic terms that could be directly measured, such as volume, temperature, or pressure. However, today the classical equation of entropy,
, was described in macroscopic terms that could be directly measured, such as volume, temperature, or pressure. However, today the classical equation of entropy, 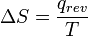 can be explained, part by part, in modern terms describing how molecules are responsible for what is happening:
can be explained, part by part, in modern terms describing how molecules are responsible for what is happening:
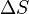 is the change in entropy of a system (some physical substance of interest) after some motional energy (“heat”) has been transferred to it by fast moving molecules. So,
is the change in entropy of a system (some physical substance of interest) after some motional energy (“heat”) has been transferred to it by fast moving molecules. So,  .
.
- Then,
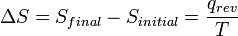 , the quotient of the motional energy (“heat”) q that is transferred "reversibly" (rev) to the system from the surroundings (or from another system in contact with the first system) divided by T, the absolute temperature at which the transfer occurs.
, the quotient of the motional energy (“heat”) q that is transferred "reversibly" (rev) to the system from the surroundings (or from another system in contact with the first system) divided by T, the absolute temperature at which the transfer occurs. - “Reversible” or “reversibly” (rev) simply means that T, the temperature of the system, has to stay (almost) exactly the same while any energy is being transferred to or from it. That’s easy in the case of phase changes, where the system absolutely must stay in the solid or liquid form until enough energy is given to it to break bonds between the molecules before it can change to a liquid or a gas. For example in the melting of ice at 273.15 K, no matter what temperature the surroundings are – from 273.20 K to 500 K or even higher, the temperature of the ice will stay at 273.15 K until the last molecules in the ice are changed to liquid water, i.e., until all the hydrogen bonds between the water molecules in ice are broken and new, less-exactly fixed hydrogen bonds between liquid water molecules are formed. This amount of energy necessary for ice melting per mole has been found to be 6008 joules at 273 K. Therefore, the entropy change per mole is
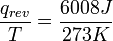 , or 22 J/K.
, or 22 J/K. - When the temperature isn't at the melting or boiling point of a substance no intermolecular bond-breaking is possible, and so any motional molecular energy (“heat”) from the surroundings transferred to a system raises its temperature, making its molecules move faster and faster. As the temperature is constantly rising, there is no longer a particular value of “T” at which energy is transferred. However, a "reversible" energy transfer can be measured at a very small temperature increase, and a cumulative total can be found by adding each of many small temperature intervals or increments. For example, to find the entropy change
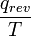 from 300 K to 310 K, measure the amount of energy transferred at dozens or hundreds of temperature increments, say from 300.00 K to 300.01 K and then 300.01 to 300.02 and so on, dividing the q by each T, and finally adding them all.
from 300 K to 310 K, measure the amount of energy transferred at dozens or hundreds of temperature increments, say from 300.00 K to 300.01 K and then 300.01 to 300.02 and so on, dividing the q by each T, and finally adding them all. - Calculus can be used to make this calculation easier if the effect of energy input to the system is linearly dependent on the temperature change, as in simple heating of a system at moderate to relatively high temperatures. Thus, the energy being transferred “per incremental change in temperature” (the heat capacity,
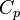 ), multiplied by the integral of
), multiplied by the integral of 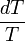 from
from 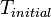 to
to 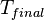 , is directly given by
, is directly given by 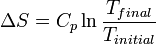 .
.
- “Reversible” or “reversibly” (rev) simply means that T, the temperature of the system, has to stay (almost) exactly the same while any energy is being transferred to or from it. That’s easy in the case of phase changes, where the system absolutely must stay in the solid or liquid form until enough energy is given to it to break bonds between the molecules before it can change to a liquid or a gas. For example in the melting of ice at 273.15 K, no matter what temperature the surroundings are – from 273.20 K to 500 K or even higher, the temperature of the ice will stay at 273.15 K until the last molecules in the ice are changed to liquid water, i.e., until all the hydrogen bonds between the water molecules in ice are broken and new, less-exactly fixed hydrogen bonds between liquid water molecules are formed. This amount of energy necessary for ice melting per mole has been found to be 6008 joules at 273 K. Therefore, the entropy change per mole is
Introductory descriptions of entropy
Traditionally, 20th century textbooks have introduced entropy as order and disorder so that it provides "a measurement of the disorder or randomness of a system". It has been argued that ambiguities in the terms used (such as "disorder" and "chaos") contribute to widespread confusion and can hinder comprehension of entropy for most students. A more recent formulation associated with Frank L. Lambert describing entropy as energy dispersal describes entropy as measuring "the spontaneous dispersal of energy — at a specific temperature."